The World Health Organization (WHO) is convening an emergency committee to address the escalating mpox outbreak, which has now impacted 116 countries. This emergency meeting comes after the WHO classified the situation as an “acute” Grade 3 emergency, the highest level of alert, signaling an urgent need for global action.
Persistent Outbreak and Global Spread
Since its emergence in 2022, the mpox outbreak has continued to spread, with recent reports indicating a rise in cases worldwide. The disease has affected countries across West, Central, and East Africa, and cases have also surfaced in the Americas and Europe. In India, the first confirmed case of mpox was reported in Kerala in 2022, affecting a 35-year-old patient.
Understanding Mpox
Mpox, previously known as monkeypox, is caused by the mpox virus, which belongs to the Orthopoxvirus genus. The virus was first identified in monkeys in Denmark in 1958, but it primarily infects rodents and other small mammals. Mpox is a rare zoonotic disease, meaning it is transmitted from animals to humans. It belongs to the Poxviridae family, which also includes viruses responsible for smallpox, cowpox, and vaccinia.
There are two primary genetic clades of mpox: Clade I, mostly found in Central and East Africa, and Clade II, which is more prevalent in West Africa. The first human case was reported in 1970 in a nine-year-old boy in the Democratic Republic of the Congo. In 2022, the WHO renamed monkeypox to mpox to reduce stigma associated with the disease.
Symptoms of Mpox
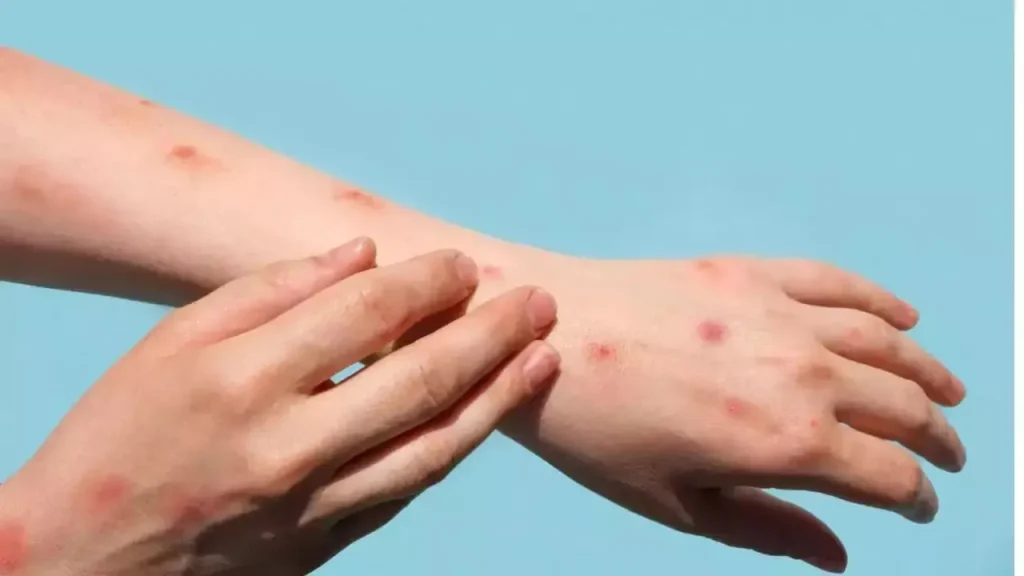
Mpox symptoms are similar to those of smallpox and include fever, headache, muscle aches, and a distinctive rash that often begins on the face and spreads to other parts of the body. The virus spreads to humans through direct contact with infected animals, bodily fluids, or contaminated materials, and can also be transmitted from person to person via respiratory droplets or close contact. Symptoms typically last between 2 to 4 weeks, with most people experiencing mild symptoms, though severe cases may require medical attention. Children, pregnant women, and those with weakened immune systems are at higher risk.
Testing and Diagnosis
The WHO recommends detecting mpox through polymerase chain reaction (PCR) tests that identify viral DNA. The most reliable samples are obtained from the rash, such as skin, fluid, or crusts, using thorough swabbing. In the absence of skin lesions, oropharyngeal, anal, or rectal swabs can be used. Blood testing is not advised, and antibody detection methods are generally not effective for distinguishing between different orthopoxviruses.
Treatment and Management

Currently, there is no cure for mpox. The WHO advises supportive care to manage symptoms. Patients should stay hydrated, consume nutritious food, get adequate rest, and avoid scratching lesions. Good hygiene practices, such as frequent hand washing, especially after touching lesions, are crucial. Infected individuals should cover their lesions with clothing or bandages and isolate from others if possible.
Vaccination and Preventive Measures
In January 2022, the European Medicines Agency approved tecovirimat, an antiviral initially developed for smallpox, for treating mpox under exceptional circumstances. However, its use is generally limited to clinical trials or expanded access programs to collect further data.
Three vaccines—MVA-BN, LC16, and OrthopoxVac—developed for smallpox are also approved for preventing mpox. Despite this, vaccination is recommended only for those at high risk, and mass vaccination against mpox is not currently endorsed by the WHO. The organization has called on manufacturers to submit Expressions of Interest for Emergency Use Listing (EUL) in response to the concerning spread of the disease.
