Neuralink, the brain-chip startup led by billionaire entrepreneur Elon Musk, has reached a significant milestone by gaining approval for its first human trial. The trial’s primary focus will be on patients who are dealing with paralysis due to cervical spinal cord injuries or amyotrophic lateral sclerosis (ALS). The announcement of this groundbreaking development was made on September 19, although the exact number of participants remains undisclosed.

A Smaller Participant Pool
Originally, Neuralink had sought approval for 10 participants. However, due to safety concerns raised by the U.S. Food and Drug Administration (FDA), the company found itself engaged in negotiations to reduce the number of participants. At present, the final number of participants approved by the FDA has yet to be confirmed. This development follows Neuralink’s previous approval for its first-in-human clinical trial in May, a time when the company was also under federal investigation for its animal testing practices.
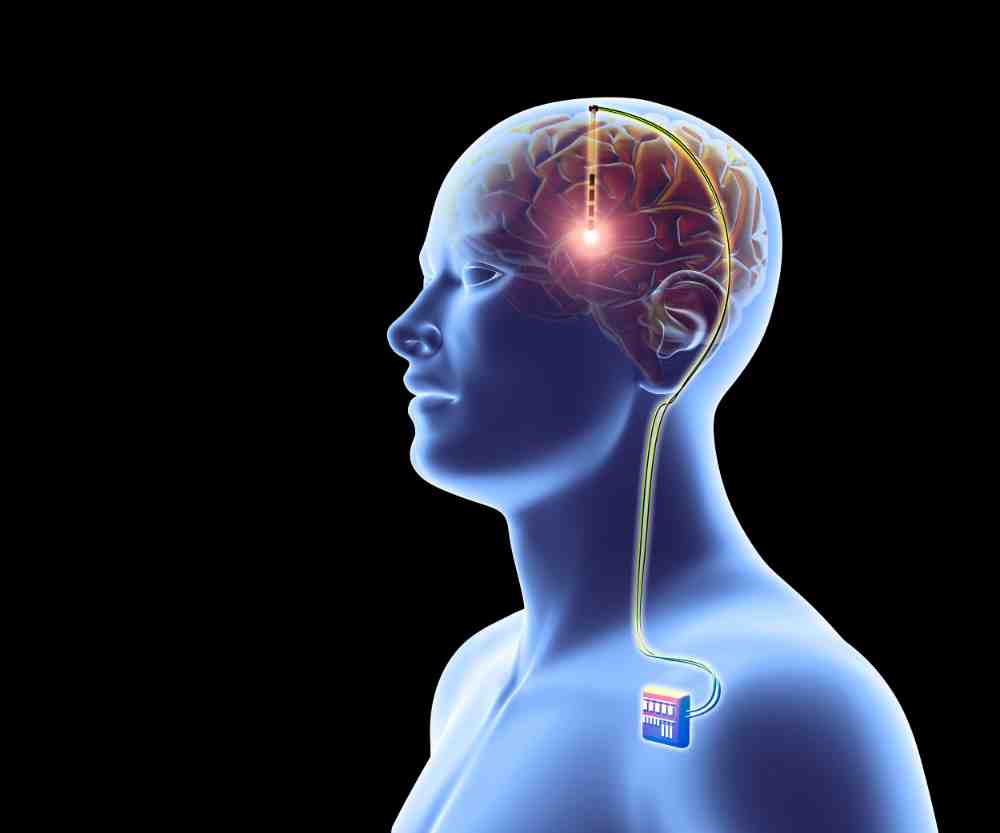
A Long Road Ahead
Experts in the field have weighed in on the timeline for Neuralink’s progress. Even if the Brain-Computer Interface (BCI) implant proves to be safe for human use, it may be a decade or more before commercial applications are authorized, according to Reuters.
Beyond Paralysis Treatment
Elon Musk’s vision for Neuralink extends far beyond the treatment of paralysis. He envisions a future where chip devices can be rapidly surgically inserted to manage a wide range of conditions, including obesity, autism, depression, and schizophrenia.
The Surgical Process
At the core of this groundbreaking trial is a complex surgical procedure. A robot will precisely place the BCI implant into a specific area of the brain responsible for the intention to move. The immediate goal is simple yet revolutionary: to enable test subjects to operate a computer keyboard or move a cursor solely by using their thoughts.
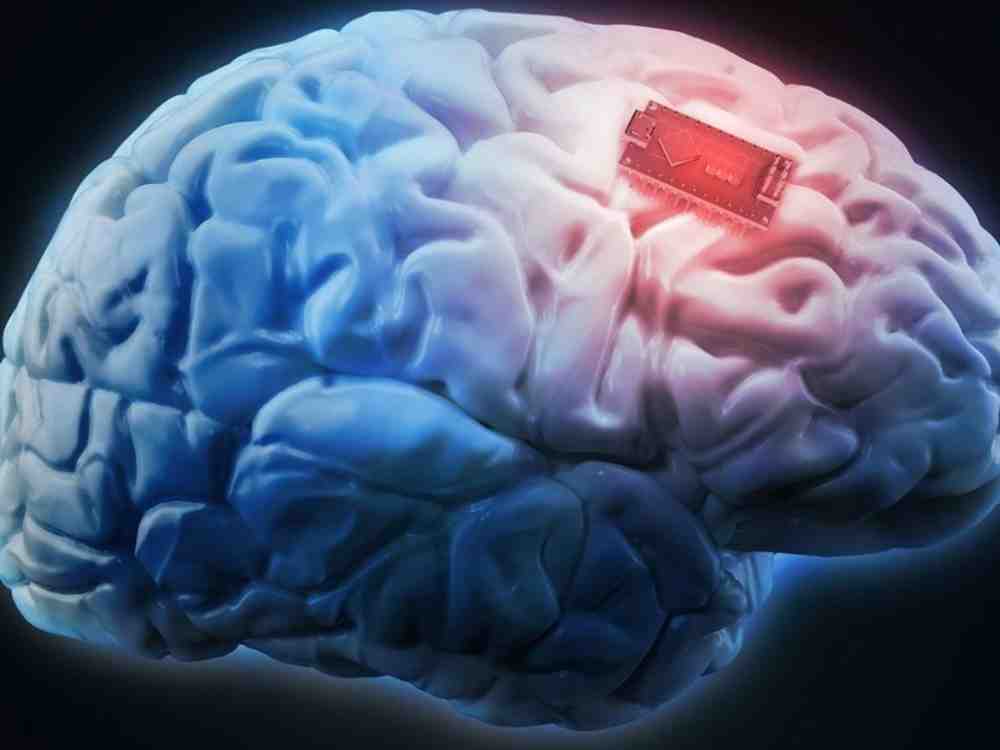
The Road Ahead
The clinical trial is expected to span approximately six years, providing ample time to thoroughly assess the safety and efficacy of the implant. While some skepticism remains, this venture adds a new dimension to the ongoing discussion of how technology can be harnessed to address complex health issues.
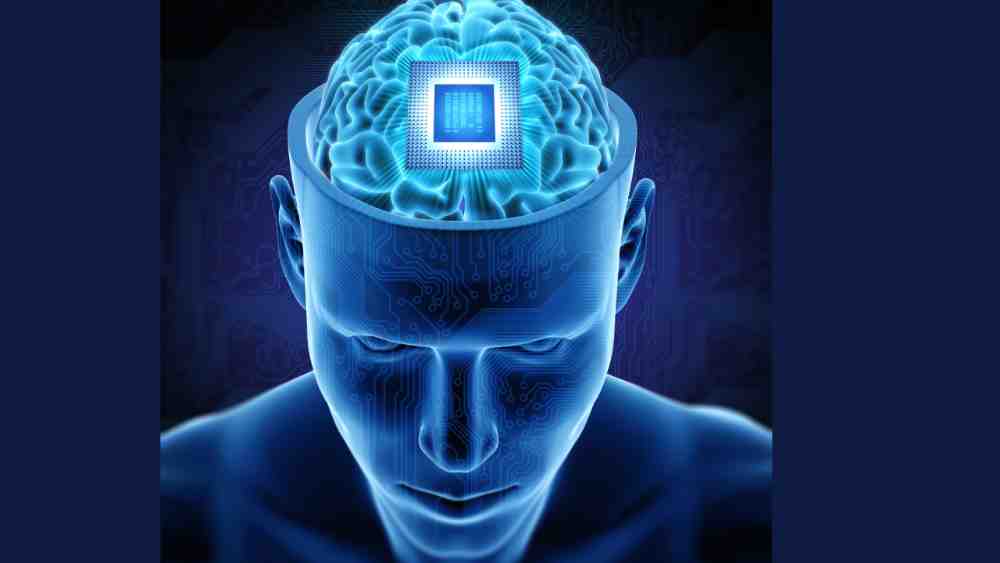
Neuralink’s pursuit of brain-chip technology is pushing the boundaries of medical science, potentially offering hope to those suffering from paralysis and paving the way for future applications in the field of neuroscience.
